Wizard Tips from the CDC
Based on what is currently known about the coronavirus and similar coronaviruses that cause SARS and MERS, spread from person-to-person with these viruses happens most frequently among close contacts (within about 6 feet). This type of transmission occurs via respiratory droplets. On the other hand, the transmission of novel coronavirus to persons from surfaces contaminated with the virus has not been documented. Current evidence suggests that novel coronavirus may remain viable for hours to days on surfaces made from a variety of materials. Cleaning of visibly dirty surfaces followed by disinfection is a best practice measure for the prevention of COVID-19 and other viral respiratory illnesses in households and community settings. Read more from the CDC
Alcohol
When referring to disinfecting agents, “alcohol” typically refers to two water-soluble chemical compounds (ethyl and isopropyl alcohol) that have generally underrated germicidal characteristics.These alcohols are rapidly bactericidal (kills bacteria) rather than bacteriostatic (stops bacteria from reproducing-but does not kill). They also kill tuberculosis, fungus, and viruses but do not destroy bacterial spores. Their disinfecting activity drops sharply when diluted below 50% concentration, and the optimum bactericidal concentration is 60%–90% solutions in water. Purell is an instant hand sanitizer made of ethyl alcohol. It has been claimed to “[kill] more than 99.99% of most common germs that may cause illness in a healthcare setting, including MRSA & VRE.” However, currently the FDA warns that the product may not be effective at eliminating diseases because there are no peer-reviewed, published clinical studies demonstrating the company’s claims.
Optical detection of Ethanol via Absorption Spectroscopy is very difficult due to weak absorption of the Ethanol molecules. Furthermore, it is very difficult to distinguish between different types of Ethanol via Absorption Spectroscopy. A more suitable detection method is Raman Spectroscopy. In the spectrum to the right you can see individual Raman spectra of ethanol and isopropanol as well as a mixture.
Chlorine and Chlorine Compounds
Hypochlorites, the most widely used of the chlorine disinfectants, are available as liquid (e.g., sodium hypochlorite) or solid (e.g., calcium hypochlorite). The most prevalent chlorine products in the United States are aqueous solutions of 5.25%–6.15% sodium hypochlorite (see glossary), usually called household bleach. They have a broad spectrum of antimicrobial activity, do not leave toxic residues, are unaffected by water hardness, are inexpensive and fast-acting.
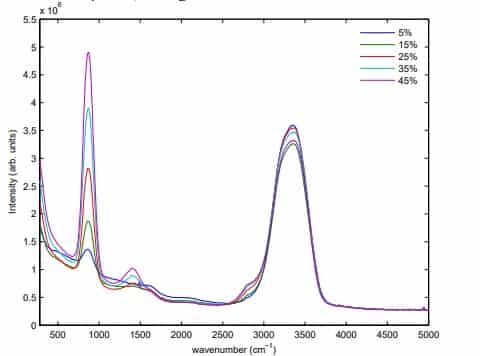
Hydrogen Peroxide
Hydrogen peroxide works by producing destructive hydroxyl free radicals that can attack membrane lipids, DNA, and other essential cell components. Commercially available 3% hydrogen peroxide is a stable and effective disinfectant when used on surfaces. It is used in concentrations that vary from 3% to 6% for disinfecting soft contact lenses (e.g., 3% for 2–3 hrs), ventilators , fabrics, and endoscopes. Hydrogen peroxide is effective in spot-disinfecting fabrics in patients’ rooms in hospitals and doctor’s offices. This is a commonly used disinfectant and can be found in many households. Hydrogen peroxide is an environmentally safe alternative to chlorine-based bleaches because it degrades to form oxygen and water.
Raman spectroscopy is a proven method for measuring hydrogen peroxide concentrations in water and the peak intensity ratio can be used for quantitative prediction. The peak at about 876 cm-1 originates from the vibration between the two oxygen atoms in hydrogen peroxide molecules. The smaller peak at about 1409 cm-1 originates from a symmetrical bending motion in the hydrogen peroxide molecules. The broad peak at about 3350 cm-1 corresponds to the symmetric vibration between the hydrogen and oxygen atom in both water molecules and hydrogen peroxide molecules.

Quaternary Ammonium Compounds
Quaternary ammonium compounds (also known as quats) are very effective disinfectants. Quats are commonly found in disinfecting sprays and wipes. Some of the common names that you might see are: alkyl dimethyl benzyl ammonium chloride, alkyl didecyl dimethyl ammonium chloride, and dialkyl dimethyl ammonium chloride. Chemically, these compounds have a common molecular structure. By changing the basic molecule scientists can change the characteristics of the entire compound. This allows quats to be used in a variety of products such as disinfectants, fabric softeners, and other cleaners. Quats are known as positively-charges surfactants. Because of the positive charge, quats can adhere to bacteria or viruses and dissolve the cell walls. Some important considerations for using quats are : concentration, application (read the product label), and contact time.
New ACS SpectraWizard Pop Sockets
The SpectraWiz also wants you to know the best way to prevent the spread of colds, flus, and other sicknesses is by washing your hands! The FDA says that “[f]ollowing simple handwashing practices is one of the most effective ways to prevent the spread of many types of infection and illness at home, at school and elsewhere.” You should wash your hands often – including before eating food, after using the toilet, after blowing your nose, sneezing or coughing, and after touching garbage. You should be washing your hands for at least 20 seconds.
If you think your hands are dirty – imagine how dirty your phone is! The SpectraWizard and Application Scientists here at StellarNet were ready to give away StellarNet popsockets to everyone who visited out booth at ACS. Because of the cancellation – all new orders will ship with a calming SpectraWiz popsocket! Contact your Application Scientist if you’d like one too!