StellarNet and the SpectraWizard wish you a Happy 4th of July!
Fun Facts about the Chemistry of Fireworks:
Fireworks require 3 types of components in order to work: an oxidizer, fuel, and a chemical mixture to create color and sound. The invention of fireworks can be traced back to ancient China, around 200 BC. Initially, bamboo stalks were thrown into fires to produce loud bangs, and later, the Chinese invented gunpowder and began using it in fireworks.
Fireworks are propelled into the air using a lift charge, usually gunpowder, which creates enough force to launch the firework high into the sky before it explodes. They rely on chemical reactions to produce their spectacular effects. When the chemicals in fireworks are ignited, they undergo exothermic reactions, releasing energy in the form of light, heat, and sound.
The oxidizer breaks the chemical bonds in the fuel, releasing all the energy at once.
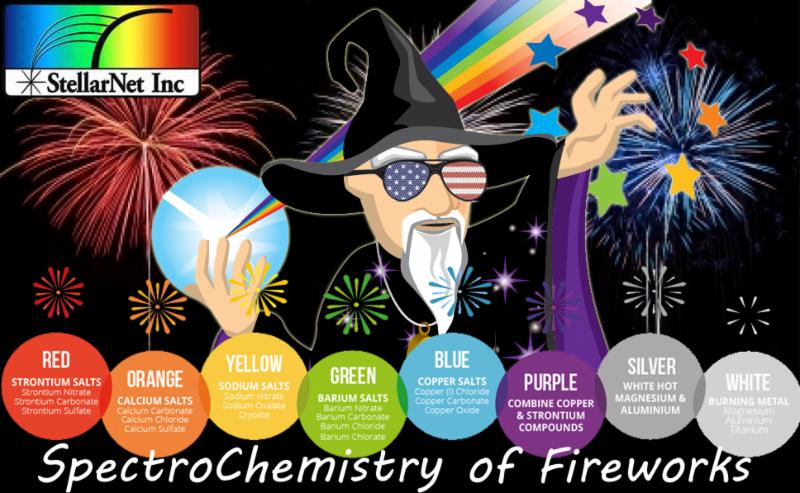
The vibrant colors in fireworks come from different metal salts. For example, strontium produces red, sodium yields yellow, barium gives green, and copper creates blue. When these metals are heated, their electrons get excited and then release energy as colored light. The shapes of the fireworks displays are determined by how the stars (small pellets of explosive material) are arranged inside the shell. For example, if the stars are arranged in a circle, the explosion will produce a circular pattern.
Fireworks are carefully timed to create specific effects. This is done by using different layers of chemicals that ignite at different times, creating a sequence of explosions. To ensure safety, fireworks are designed with specific burn rates and delay fuses to control the timing of the explosions. The outer shell of the firework also helps contain and direct the explosion.
The loud booms and crackles are due to the rapid expansion of gases. When the fireworks explode, the gases expand rapidly and create shock waves, which we hear as sound.
-
- When Potassium perchlorate and sodium salicylate burn, they slowly release gas, creating the whistling sounds commonly heard in rockets.
- Titanium powder can be incorporated in mortars to make load blasts.
- Aluminum or Iron flakes can be used to make a hiss or a sizzle.
To celebrate the holiday, the SpectraWizard and the StellarNet team wanted to take emission spectra of some of America’s favorite fireworks! It was a BLAST 🙂
Firework emission spectra can show us the color of the firework itself as well as the specific energy frequency released as the reactive metals produce oxides. The metals below give off the respective emission color:
|
Strontium
|
Calcium
|
Sodium
|
Barium
|
| Copper |
Strontium
|
Magnesium
|
Titanium |