Sean Grabe, Matthew Dent, Shumaila Babar, Teng Zhang, Stephen Tennison, John F. Watts, and Constantina Lekakou
Published: 28 February 2023 | Published on behalf of The Electrochemical Society
Abstract
A major challenge in the simulation of Li-S batteries is that the electrochemical reaction parameters supplied from the fitting of 0-d or 1-d models depend on the cathode and separator microstructure, so these parameters cannot be used in the design and optimization of material microstructure. The present investigation fits the electrochemical reaction kinetics employing a continuum model taking into account the pore size distribution and tortuosity of cathode and separator in the transport of sulfur molecules and ion species (Li+ ion, electrolyte and sulfide anions) in solvated or desolvated form depending on pore size. Hence, the specified reaction kinetics parameters are independent from the material microstructure. The Li-S redox reaction model includes six redox reactions in the cathode and the lithium redox reaction at the anode. Reactions are assumed to take place in the electrolyte solution rather than in the solid phase of sulfur or lithium sulfide precipitates, where dissolution/precipitation kinetics is modeled especially for the low solubility compounds: sulfur, Li2S and Li2S2. The fitting exercise is conducted based on experimental data of a cyclic voltammetry cycle accompanied by in operando UV–vis spectroscopy. The investigated battery has electrolyte LiTFSI in DOL/DME and no electrocatalysts in any part of the cell.
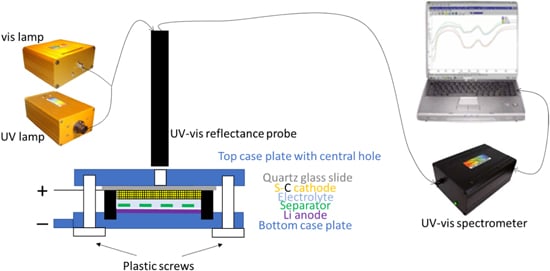
… At the same time in operando UV–vis spectroscopy was conducted on the cathode window every 10 min using a StellarNet system consisting of a portable BLK CXR UV–vis spectrometer with 273–900 nm range, a SL1-SL3 Combo deuterium and halogen light source for 200–2300 nm range, and a R600–8-UVVIS-SR reflectance probe on a stand (see diagram in Fig. 3) …





