Abstract
The indoor environment provides unique surfaces and lighting conditions which affect the photochemistry taking place there. As indoor illumination sources typically output wavelengths too long to affect gas phase photochemistry, the potential for surface photochemistry induced by indoor light sources has been mostly unexplored. In this proof of concept study, we report the emission of gas phase nitrogen oxides as a product of the illumination of glass surfaces coated with nitrate-doped TiO2 and nitrate deposited on indoor paint, using a variety of common indoor light sources. Fluorescent, incandescent, halogen, and LED lights were studied, and a xenon lamp was used for baseline measurements. NOx was emitted from all samples, thus establishing that renoxification can occur in indoor environments. NO2 (g) was the predominant species emitted from samples coated with nitrate-doped TiO2, and NO (g) was the predominant species emitted from nitrate-deposited painted glass surfaces. It was also found that heating from the light sources had no effect on the production of NOx. This preliminary study establishes the potential for heterogeneous photochemistry to occur on real indoor surfaces and opens the way for further research to be conducted under realistic indoor conditions.
Introduction
The oxidizing nature of the atmosphere influences the chemical processing of atmospheric constituents which in turn affects atmospheric air quality. Atmospheric oxidation can be initiated either photochemically or via reaction with the hydroxyl radical (OH•), the nitrate radical (NO3•), or ozone (O3). Outdoors, tropospheric OH• is primarily produced via the photolysis of ozone in the wavelength range from 295 to 320 nm. Since indoor light sources typically emit light at wavelengths greater than 320 nm, it has been predicted that OH• concentrations indoors should be orders of magnitude lower than those observed outside. However, hydroxyl radical concentrations of 106 molecules cm−3, comparable to outdoor urban summertime concentrations, have been measured in indoor environments. It has been suggested that these high OH• concentrations are due to the photolysis of nitrous acid (HONO), which can occur at wavelengths less than 400 nm. Although gas phase HONO has been observed indoors, a thorough understanding of its production mechanisms is still lacking. Read publication…
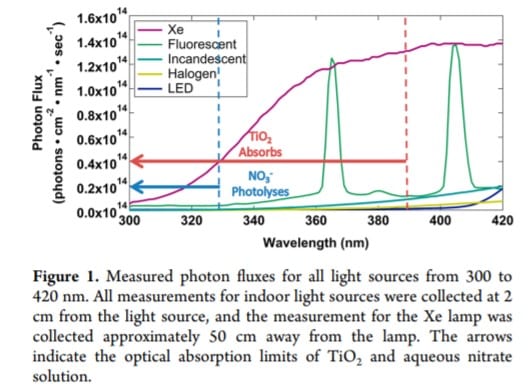
Characterization of Indoor Light Sources.
The following illumination sources were used in this study: Phillips Energy Saver 60W replacement 13W fluorescent bulbs, General Electric 60W incandescent bulbs, Sylvania 60W replacement 43 W halogen bulbs, Sylvania 60W replacement 8.5W indoor/outdoor LED bulbs, and an Oriel 150W xenon lamp. All bulbs that were used were “soft white”. The photon flux of each light source was measured using a StellarNet BLACK-Comet spectrometer and is illustrated in Figure 1 for the wavelength range 300−420nm. Our results agree well with those presented in Kowal. The flux was recorded at 2cm from the light source for all bulbs except the xenon lamp which was recorded at a distance of 50cm owing to the geometry of the lamp. The purpose of this experiment was to determine whether gaseous NOx could be emitted from surfaces via heterogeneous photochemistry induced by indoor illumination. In order to maximize the potential chemistry in this proof of concept experiment, 2cm was selected as the distance between the majority of the light sources and the sample. Typically, indoor distances between illumination sources and surfaces will be much larger, and the resulting decrease in light intensity should be taken into account. However, this was not the aim of the present study. Go to StellarNet Light Measurement Systems
Results and Discussion
In the majority of cases, sample illumination with indoor light sources produced NOx and HONO. These concentrations were calculated by allowing the gaseous concentrations to stabilize and then averaging the stable concentrations over time. For the purposes of this study, it is the relative concentrations of different NOx species that are of interest in order to compare the effects of different illumination sources and surface types. Figure 2 illustrates the time dependence of production of NOx and its subsequent stabilization when a TiO2 slurry sample is illuminated with an incandescent bulb. Read publication…





